REGULATORY AFFAIRS MANAGEMENT
CREATION OF AN
MDR | IVDR ROADMAP
GAP Analysis
Formulate Intended use & performance promises
Classify products
Implement QM system, UDI system
IMPLEMENTATION OF LEGAL REQUIREMENTS
Creation of the product file
according to MDR/IVDR, national and regional regulations
Classify products according to MDR/IVDR classification rules
Selecting the conformity assessment procedure
Implement clinical evaluation requirements according to MEDDEV 2.7/1 Revision 4
Implement post-market surveillance PMS
IMPLEMENTATION OF GSPR
Proof of the general safety and performance requirements through harmonised standards and state of the art
ISO 13485 Medical devices – Quality management system
ISO 14971 Application of risk management
IEC 62304 Software life-cycle processes
IEC 62304 Health software
IEC 62366-1 Application of usability engineering
IEC 60601-1
Medical electrical equipment – General requirements for basic safety and essential performance
MEDICAL ENGINEERING
ELECTRICS | PNEUMATICS
Creation of normative documents
- Functional diagrams
- Block diagrams
- Insulation diagrams
- Wiring, pneumatic and hydraulic diagrams
MECHANICS
Creation of CAD construction drawings
- Prototype drawings
- Production drawings
- Assembly drawings
SOFTWARE
Software developement
- Recording of stakeholder requirements
- Support with software development
- Documentation according to required regulations
SAFETY INSPECTION
According to the MDR and national regulations, dental and medical devices must be inspected recurrently, between 12 and 36 months.
The frequency of the inspection depends on the type of device and the manufacturer’s specifications.
MAINTENANCE & REPAIR
The devices are maintained and checked on site, taking into account your daily procedures.
We will support you by suggesting solutions to rectify any defects found.
All repairs are carried out promptly and in compliance with all normative requirements.
INSPECTION ACCORDING TO
IEC 62353
Medical electrical equipment
MDR
Medical Device Regulation
National regulations
CUSTOMERS
My customers are manufacturers of dental and medical devices, physiotherapy centers and dental clinics in Austria and Germany.
RECOMENDATIONS
DEVELOPEMENT PROJECT
W&H test device for dental motors and air instruments
CONSULTING
Innodent TM6+ dental treatment unit
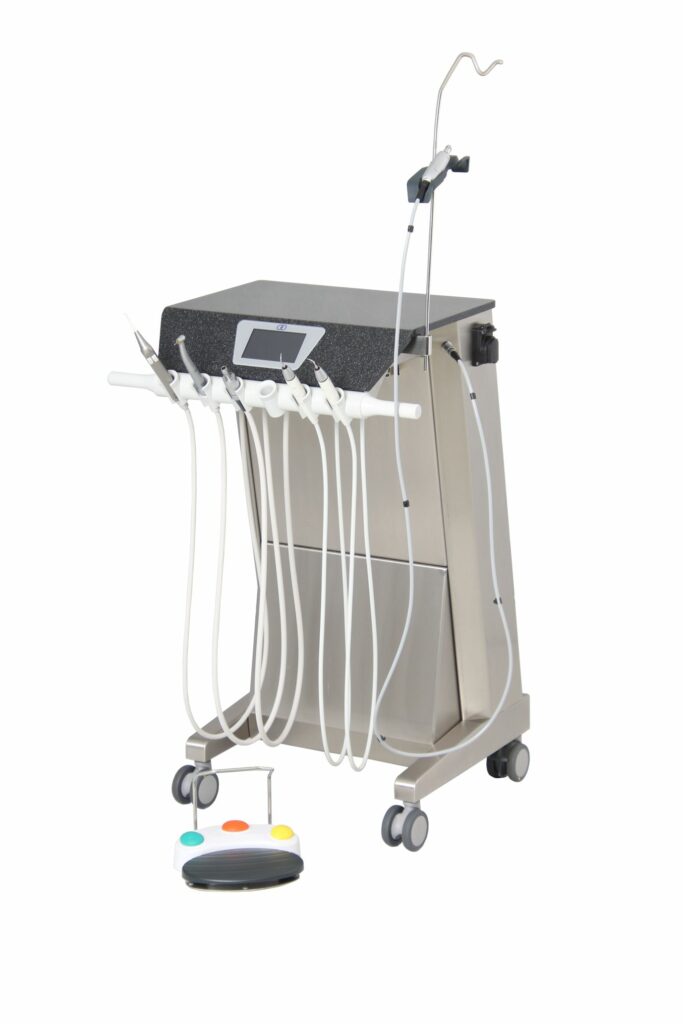
CONSULTING
Innodent PowerSuc dental suction unit

- Creation of a project roadmap
- Requirements documentation
- Product design
- Realisation of technical and legal requirements
- Design transfer
- Creation of an MDR roadmap
- Implementation of legal requirements
- Proof of GSPR
- Creation of normative required documents

- Creation of an MDR roadmap
- Implementation of legal requirements
- Proof of GSPR
- Creation of normative required documents
My goal is not only comprehensive advice and support, but also optimal pricing.
CONTACT
You can reach us by phone







